CLINICAL TRIAL SERVICES

Study set-up
Consulting QuantifiCare during the early stages of development will provide valuable insight into the best techniques, practices and imaging technologies to achieve efficacy data in your study. Whether you are designing a single-center or a large multi-center clinical trial, it is critical that a standardized approach is taken to ensure excellent quality, reproducible images.
All photography devices are portable, user-friendly and rely on a ranging light system to ensure consistent, high-quality images at each investigative sites and across all patient visits.
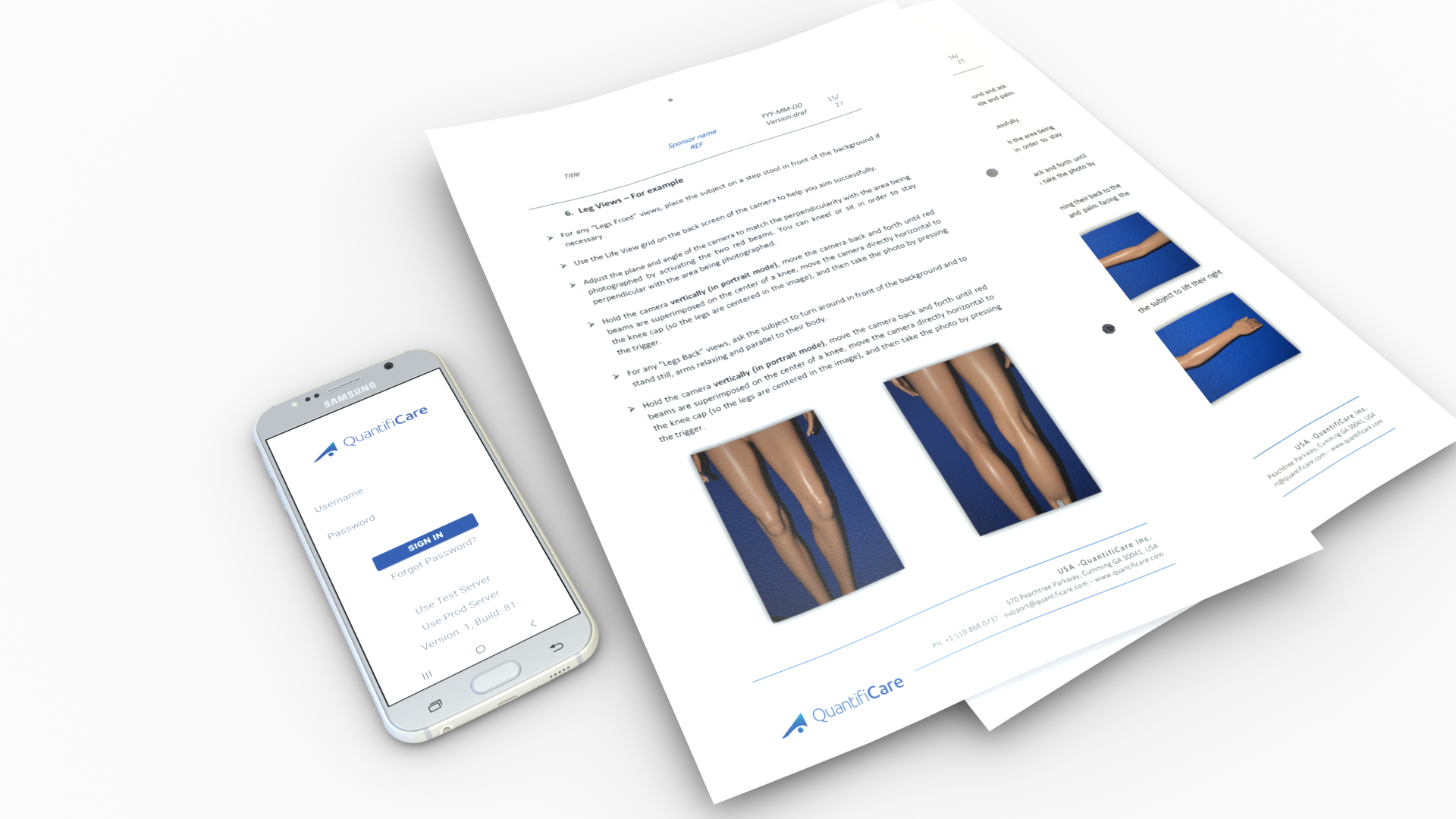
A study-specific imaging procedure is designed and documented as part of the Photography Binder provided to the investigative sites involved in the study.
This reference document is used for the training of clinical staff and includes a step-by-step guide for the capture of digital photographs, as well as details on the upload, monitoring and quality control requirements.
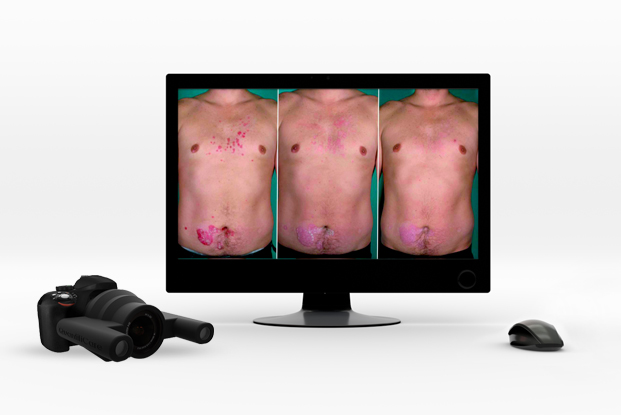
QuantifiCare has developed a full range of innovative standardized camera systems to document and assess treatment response, and deliver objective results to support your claims.
2D DermaViz® Series
The 2D DermaViz® is a fully handheld photography device equipped with fixed optics and a built-in ranging light system, ensuring high-quality, reproducible images. Designed for clinical staff use, this system is easy-to-use and delivers consistent results regardless of the extent of previous experience in photography.
3D LifeViz® Series
The 3D LifeViz® Series offers a range of high-quality 3D stereoscopic systems to consistently document any part of the body with unmatched accuracy and precision.
All of our 3D systems are fully handheld and rely on a built-in ranging light system to ensure high-quality, reproducible images. Combined with our image analysis services, the 3D LifeViz® systems offer a powerful solution to objectively evaluate treatment response and support your claims
The training of the investigative site staff is critical to successfully capture high quality reproducible trial images. QuantifiCare offers several training options, including remote, on-site, investigator meeting and custom video
Upon completion of the training, each investigative site staff is required to pass a qualification procedure. During this step, photographers must simulate two mock visits following the study Image Procedure. Qualification images are then uploaded to DermaViz® Web Portal, and controlled for quality and reproducibility. Investigative sites will only be permitted to enter the study once they have been qualified.

In-Study Operations
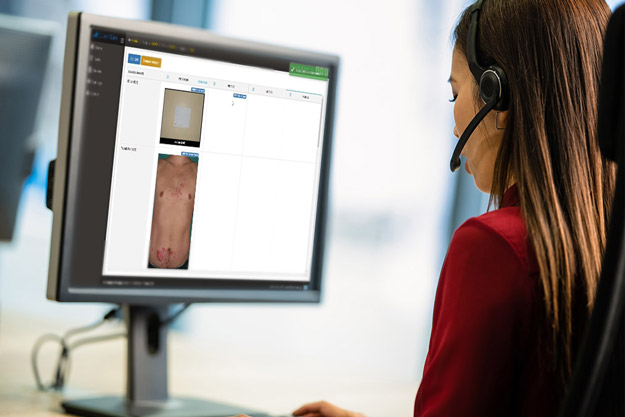
QuantifiCare’s innovative Web Portal bridges the gap between traditional paper solutions and e-CRFs while complying with electronic standards required by regulatory agencies. Backed by an experienced cross-functional team, ready to provide support at each step of the way to ensure a seamless experience for Sponsors, Investigators and Patients alike.
Forget time-intensive imaging software. Keep your investigators happy and enhance their imaging workflow with a comprehensive web solution.
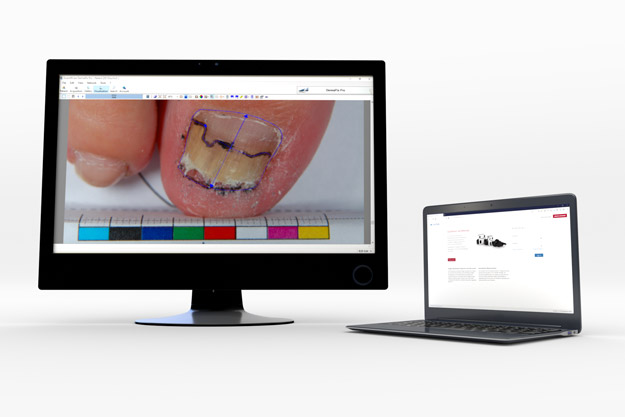
DermaPix® Web Portal is the end-to-end solution for the upload, management, monitoring and real-time review of digital images in multicenter clinical trials.
Securely upload study images in a few simple steps.
A customizable interface to easily classify images by patient, visit and region of interest
Track and review study images in real-time as they are uploaded by the sites
Easily keep track of open queries to ensure compliance with imaging standards
A simple, automated way to streamline data collection
A record of all data and actions performed on DermaPix Web Portal is kept as part of our audit log
QuantifiCare is committed to providing the highest standards of quality and compliance to ensure robust efficacy data is achieved for your study. Our QA team is responsible for ensuring that our Standard Operating Procedures (SOPs) are maintained by our clinical team members to ensure regulatory compliance from study initiation to final database lock.
Our DermaPix Web- Portal provides a high range of tools to comply, maintain and monitor compliance throughout the duration of the study.
- ✓ GCP, FDA 21CFR Part11 & HIPAA compliant: electronic signatures, time-stamps, fingerprints, traceable audit records.
- ✓ Secured electronic data transmission, automated edit checks (CRC) for clean data transfer
- ✓ Protected data storage both on-site and at QuantifiCare’s twin data centers
- ✓ Provide Secured web access to centralized pictures
- ✓ Standard15-year archiving of study data





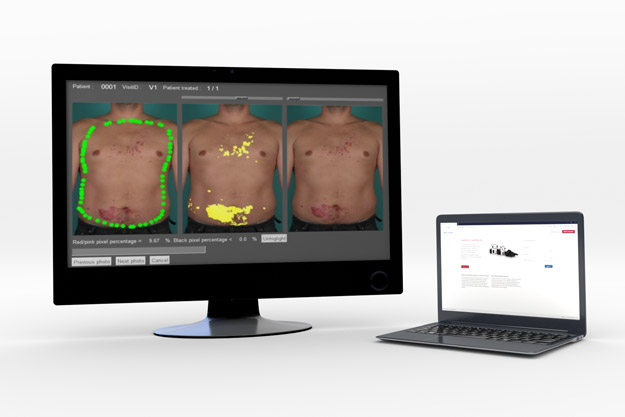
Image Analysis
Our founders are all renowned experts in image processing, and have continuously advocated for imaging research by applying their expertise to the development of innovative solutions for imaging endpoints in clinical studies.
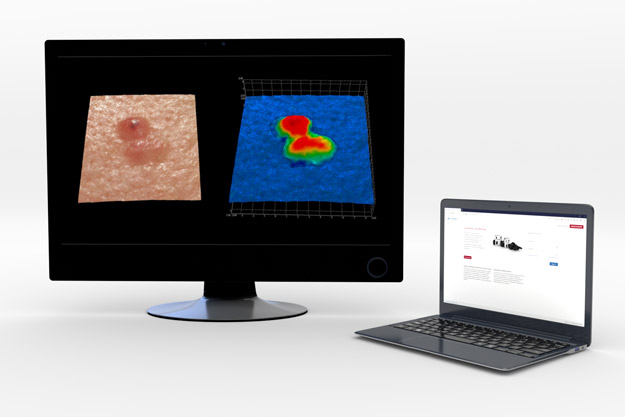
QuantifiCare offers central and remote image analysis services to support inclusion/exclusion screening criteria and imaging endpoints. Our solution relies on both validated systems and documented methodologies which are customized to meet your protocol requirements.
Over the past two decades, QuantifiCare has had the privilege of working with and establishing strong partnerships with numerous Key Opinion Leaders in core therapeutic areas.
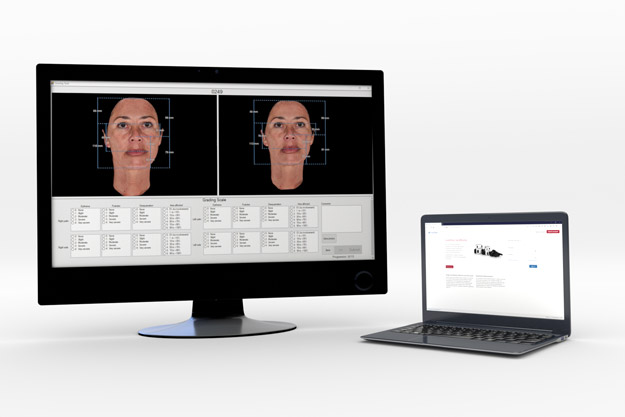
Independent Panel Reviews
Independent Panel Reviews (IPRs) are an effective, unbiased way to measure treatment efficacy.
In some cases, on-site expert panels may be too difficult to organize due to time and agenda constraints. Remote centralized reviews of imaging data can help bypass these costly logistical hurdles while reducing the variability and bias.
QuantifiCare offers custom-formatted reading tools, providing experts with a secure remote access to perform blinded, randomized review of study images.
Our customizable IPR tools can easily integrate validated scales and offer the ability to perform real-time inclusion/exclusion testing for patient screening.